
Non-Cystic Fibrosis Bronchiectasis
Zambon is committed to developing novel treatments for severe respiratory diseases such as NCFB
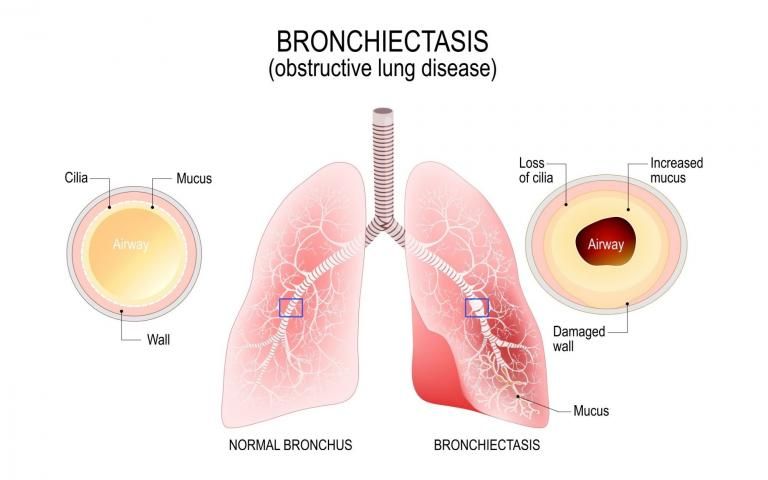
NCFB
Non-cystic Fibrosis Bronchiectasis (NCFB) is a serious respiratory disease characterized by chronic inflammation, wall thickening and dilatation of the airways, with an estimated 50%-80% of cases having no clear cause.1 Patients suffer from chronic cough, increased sputum production, breathlessness and fatigue. Those with this disease are much more susceptible to frequent lung infections. NCFB is often related to pre-existing chronic obstructive pulmonary disease (COPD), asthma and primary ciliary dyskinesia disease.
Understanding NCFB
NCFB is often related to pre-existing chronic obstructive pulmonary disease (COPD), asthma and primary ciliary dyskinesia disease.
In addition to adversely affecting the quality of life of patients, NCFB also imposes a significant economic burden to patients and the healthcare system.
- Real world data has shown that in addition to clear impacts to quality of life, patients with NCFB have higher mortality rates with respect to the rest of the population.2
- Traditional clinical studies evaluating long-term inhaled antibiotics have been very challenging and none have resulted in approved treatments.3
NCFB patients may develop Pseudomonas aeruginosa lung infection during their disease. P. aeruginosa bacteria in NCFB patients is associated with accelerated decline of lung function and increased risk of death, hospital admission and exacerbation.4 NCFB patients with chronic P. aeruginosa infections, represents a large unmet need in the area of chronic respiratory diseases.4
1. O`Donnell AE, Bronchiectasis, Chest, 2008;134: 815-823.
2. Quint, JK, et al. Eur Respir J. Jan 2016; 47(1): 186-193. doi: 10.1183/13993003.01033-2015.
3. Maselli DJ, et al. Int J Mol Sci. 2017;18(5):1062. doi:10.3390/ijms18051062.
4. Finch S, et al. Ann Am Thorac Soc. 2015; 12(11): 1602-11
of patients with bronchiectasis have a chronic infection
patients involved in Promis 1 and Promis 2 clinical trials
Looking into the “I”s of NCFB
NCFB experts discuss the current landscape of NCFB pathophysiology, diagnosis, treatment strategies, and future directions.
The webinar is intended for healthcare professionals
A Deep Dive into the “I”s of Non-cystic fibrosis bronchiectasis

Experts in non-cystic fibrosis bronchiectasis (NCFB) will discuss current challenges in diagnosis and management, characterization of disease severity, and emerging treatment strategies for severe NCFB.
The webinar is intended for healthcare professionals.
PROMIS Program
The PROMIS Phase 3 program is evaluating inhaled colistimethate sodium administered via the I-neb® Adaptive Aerosol Delivery system in patients with NCFB chronically colonized with P. aeruginosa to reduce the frequency of pulmonary exacerbations. The rationale of inhaled therapy is to deliver sufficient concentrations of drug directly to the site of disease while minimizing systemic exposure.
The PROMIS program consists of two Phase 3 clinical studies, PROMIS I and PROMIS II. The studies are expected to enroll about 800 patients in 17 countries, including 30 US clinical sites.
Colistimethate sodium has received a qualified infectious disease product (QIDP) and Fast Track designations from the US food and Drug Administration for the treatment of NCFB, reinforcing Zambon’s commitment to developing a treatment for patients and physicians for this debilitating disease. If approved, colistimethate sodium would be the first FDA approved treatment indicated for patients with NCFB chronically colonized with P. aeruginosa. Upon receiving necessary regulatory approvals, we are preparing market access and product launch plans in the U.S., EU and other global markets.
Expanded Access or Compassionate Use
At this time, Zambon does not offer access to our unapproved medicines via expanded access or compassionate use and believes that participating in its ongoing or future clinical trials is the best way to access this medicine that is not yet approved by U.S. Food and Drug Administration. We have considered many factors, including our ability to maintain supply for the ongoing and planned clinical trials, enroll and complete ongoing and upcoming clinical trials, and make reasonable assessments of potential risk versus benefit for patients outside the clinical trial setting. In the event that Zambon decides to consider expanded access use for inhaled colistimethate sodium administered via the I-neb® Adaptive Aerosol Delivery system in the future, this guideline will be updated and we will evaluate and respond to each expanded access request on a case-by-case basis using criteria that ensures such requests are considered in a fair and consistent manner.
If you or a family member are/is interested in gaining access to our investigational therapy, we encourage you to consult with your physician regarding the possibility of participating in our clinical trials. For additional information about this posted guideline or if you are a treating physician requesting expanded access, please contact us via email at respiratory.patients@zambongroup.com. We anticipate acknowledging receipt of such inquiries sent to this email within three business days.
The posting of this guideline by Zambon shall not serve as a guarantee of access to any specific investigational drug by any individual patient. In accordance with the 21st Century Cures Act, Zambon may revise this expanded access guideline at any time. The guideline will be updated with a hyperlink or other reference to the expanded access record on clinicaltrials.gov after such record becomes active.
