
BT-L-CsA-301-SLT
BT-L-CsA-302-DLT
BT-L-CsA-303-FU
NEWSLETTER NO. 11
This newsletter is the second in our mini-series featuring interviews of members of our BOSTON Clinical Program. In this interview, Paola Castellani, MD, Zambon’s Global Chief Medical Officer and Patient’s Access Head, discusses the BOSTON studies and their importance.
Our good science to address the disease and our studies

A CHAT WITH
Paola Castellani
Clinical Development Programs
Why is the work we are doing in BOS important?
Finding a safe and effective treatment for bronchiolitis obliterans syndrome (BOS) is urgent and of utmost importance to people who develop this devastating disease. BOS most commonly affects people who have received lung or allogeneic hematopoietic stem cell transplant (alloHSCT). Currently, there is no approved treatment indicated for BOS.1 Although the number of lung transplantations is increasing and there has been progress in overall survival, unfortunately, long-term survival has not improved in comparison to other organ transplantations. An estimated 50% of lung transplant patients develop BOS within five years post-transplant2 and BOS usually leads to respiratory failure and death within 2-4 years of diagnosis.3 The work we are doing in BOS is important since, if approved, it would be the first treatment indicated specifically for people who develop this debilitating and fatal disease.
What are the key elements of our pipeline in BOS?
Our clinical research studies for BOS are referred to as the BOSTON program. There are five studies in our BOSTON development program:
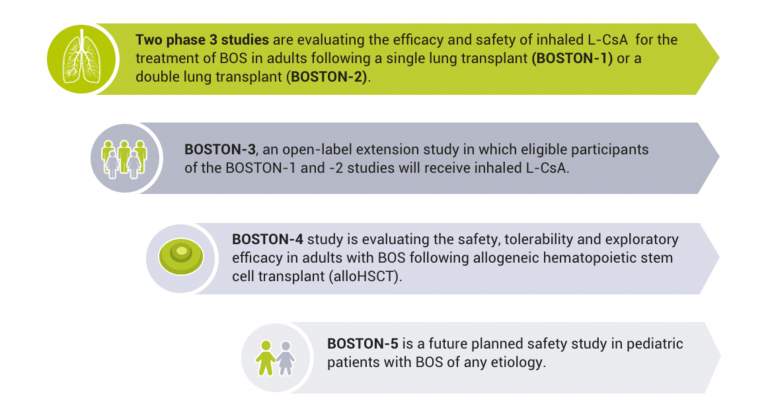
What is the main goal of the BOSTON program?
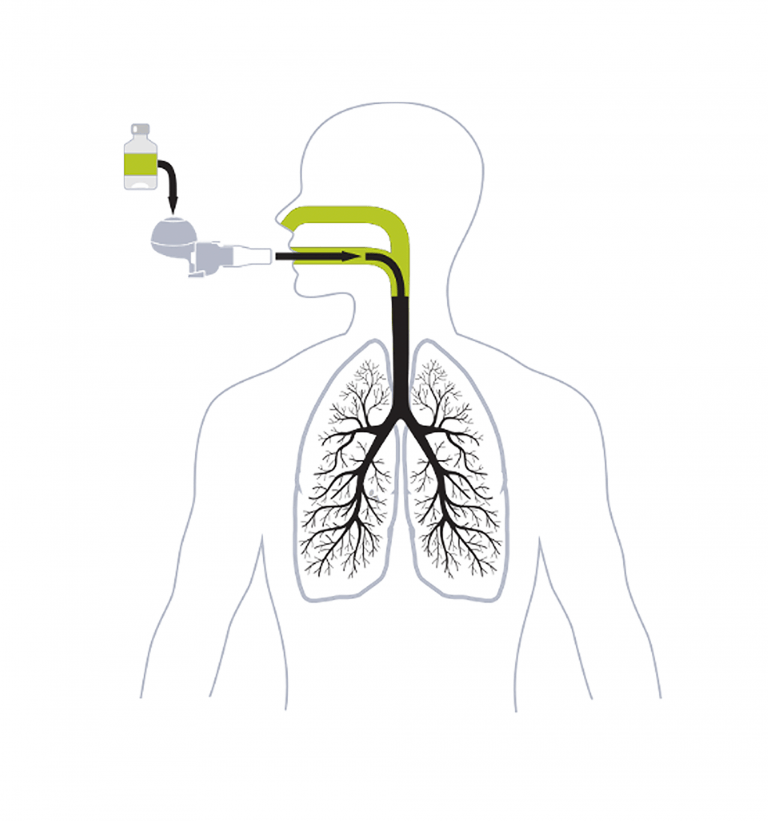
The main focus of our BOS studies is to evaluate the safety and efficacy of investigational inhaled Liposomal Cyclosporine A (L-CsA) for the treatment of Bronchiolitis Obliterans Syndrome.
Where are the studies being conducted?
BOSTON-1 and -2 studies are underway at leading lung transplant centers in 8 countries, including the US. BOSTON-4 is currently enrolling alloHSCT recipients with BOS in Germany, France and Spain.
How do you select the centers for the studies?
Our site selection involves a rigorous review process. Clinical sites participating in the BOSTON-1 and -2 studies are leading lung transplant centers worldwide and our principal investigators include some of the most renowned key opinion leaders in the field. In addition to selecting the most suitable site, it is also important to determine if the center has enough personnel and adequate time to fully participate in the study.
1. Lund LH et al. J Heart Lung Transplant. 2017; Oct; 36(10):1037-1079.
2. Weigt SS et al. Semin Respir Crit Care Med. 2013;34(3):336–351.
3. Chambers DC et al. J Heart Lung Transplant.
How Do You Like the Newsletters?
Feedback (positive or constructive) is welcome at boston.sitesupport@zambongroup.com.
Help Needed, Questions or Suggestions?
The development program for inhaled L-CsA for the treatment of bronchiolitis obliterans is very important to Zambon.
LabCorp is responsible for the operational conduct of the studies and your LabCorp contact should be your first point of contact for questions.
However, please do not hesitate to get in touch with us directly if you’d like. Emails addressed to boston.sitesupport@zambongroup.com will reach the Zambon study team and will be answered promptly.
Newsletters are for BOSTON clinical site personnel only and not intended to be shared with patients, trial participants or any external audiences.
