
BT-L-CsA-301-SLT
BT-L-CsA-302-DLT
BT-L-CsA-303-FU
NEWSLETTER NO. 12
We’ve listened and new protocol amendments are on the way!
After discussions with some of you, we have made changes to the BOSTON-1, -2, and -3 protocols that we hope will help with enrollment. Changes include:
- Lowering the FEV1 inclusion limit to include the complete range of patients diagnosed with CLAD-BOS stage 2
- Allowing the inclusion of patients diagnosed with CLAD-BODS more than than 12 months prior to screening (in which case patient must have shown a decline in FEV1 ≥ 200ml in the previous 12 months before screening, which is not due to acute infection or acute organ rejection).
- Since vaccinations against COVID-19 have started, these of course are allowed as part of standard of care
More detailed information will be shared by Covance shortly. We hope to see the amendments approved and implemented soon.
Patient Recruitment Updates
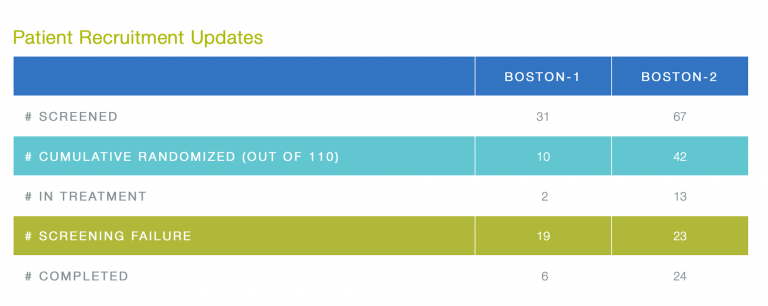
Kudos for new patient recruitment go to the following sites:
- 706 Cifrian BOSTON 2 on 17 Nov 2020
- 501 Behr BOSTON 2 on 03 Dec 2020
- 703 de la Torre BOSTON 1 on 14 Dec 2020
- 402 Reynaud BOSTON 2 on 16 Dec 2020
- 114 Arcasoy BOSTON 2 on 13 Jan 2021
- 701 Monforte BOSTON 2 on 13 Jan 2021
- 116 Huang BOSTON 2 on 13 Jan 2021
- 501 Behr BOSTON 2 on 14 Jan 2021
- 101 Griffith BOSTON 2 on 19 Jan 2021
- 103 Naik BOSTON 2 on 27 Jan 2021
New to the Team – we’d like to introduce a couple of new team members from our side

Jessica Gregory, BS RRT – Clinical Site Liaison for the US.
Jessica joined the Zambon team at the beginning of January as US Clinical Site Liaison. In her role as Clinical Site Liaison she will be working to improve and foster the relationships with clinical sites. Jessica lives in Durham, NC. She has an educational background in respiratory therapy. Jessica will be completing her Master of Science in Clinical Research and Product Development from the University of North Carolina at Wilmington this Spring. She has 7 years’ experience in Clinical Research and has worked for both Sponsors and CROs. In addition, she has 5 years’ experience at Duke University Hospital as an Advanced Respiratory Care Practitioner. We hope you will meet her soon either “online” or in person. In her free time, Jessica enjoys hiking and rock climbing in the NC mountains.

Archana Patel, RRT-ACCS – Device Trainer, Clinical Services
Archana Patel is a Registered Respiratory Therapist. She recently joined Zambon’s clinical service team as device trainer for the US. Archana brings her experience as Respiratory Care Practitioner and clinical research specialist. She also worked on the previous pilot L-CsA-i research study. She will be responsible for the device trainings at the US BOSTON sites. She may be seen online doing device training from her home office in Baltimore, MD. In her free time, she enjoys doing yoga and recently received her yoga instructor certification.
The study sponsor has changed to Zambon S.p.A.
As you know, Breath Therapeutics was acquired by Zambon SpA in 2019. As a next step of company integration, sponsorship for the BOSTON studies has been formally transferred to Zambon. This does not change anything for you regarding the study team or your contacts. You will receive more information on changes to the informed consents, labels, etc. shortly.
How Do You Like the Newsletters?
Feedback (positive or constructive) is welcome at boston.sitesupport@zambongroup.com.
Help Needed, Questions or Suggestions?
The development program for inhaled L-CsA for the treatment of bronchiolitis obliterans is very important to Zambon.
LabCorp is responsible for the operational conduct of the studies and your LabCorp contact should be your first point of contact for questions.
However, please do not hesitate to get in touch with us directly if you’d like. Emails addressed to boston.sitesupport@zambongroup.com will reach the Zambon study team and will be answered promptly.
Newsletters are for BOSTON clinical site personnel only and not intended to be shared with patients, trial participants or any external audiences.
