
BT-L-CsA-301-SLT
BT-L-CsA-302-DLT
BT-L-CsA-303-FU
NEWSLETTER NO. 14
Status Updates
After a long period, where almost no travel was possible, we as study sponsor had our first post-COVID, in-person, visit at Dr. Brian Keller’s site at the Ohio State University in Columbus, Ohio. Team members from Zambon were able to join the CRA for a re-training visit. A special thank you to Dr. Keller and team for making accommodations that allowed us to have a productive meeting while maintaining proper social distancing.
Investigator Meetings for Study Protocol Version 4
You probably do already know that a new version of the study protocol has recently been released. We do hope, that the changes will make more patients eligible for the study. There will be two Investigator Meetings for the US and one for the EU at the end of March for introducing the new protocol. In addition, we will share information on study status, best practice and more. Stay tuned for information on days and times and the agenda!
Frequently Asked Questions
We have received questions regarding inclusion/exclusion from multiple sites and want to provide clarity:
Inclusion #5 states: Patients should be on a maintenance regimen of immunosuppressive agents including tacrolimus, a second agent such as but not limited to MMF or azathioprine, and a systemic corticosteroid such as prednisone as third agent. The regimen must be stable within 4 weeks prior to randomization with respect to the therapeutic agents.
Our medical monitor has clarified that the agent must be stable, not the dose.
Per Exclusion #3, the DSA results do not need to be zero and detectable levels are acceptable, if patients are judged clinically stable (by investigator).
If you have any questions please do not hesitate to reach out, as it is likely that someone else has the same question.
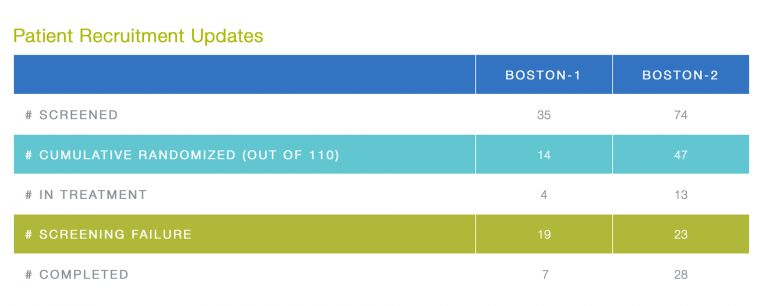
Kudos for new randomizations go to the teams of the following sites:
- 116 Huang BOSTON-1 on 5 Feb 2021
- 502 Gottlieb BOSTON-2 on 10 Feb 2021
- 109 Pilewski BOSTON-2 on 12 Feb 2021
- 701 Monforte BOSTON-2 on 24 Feb 2021
- 114 Arcasoy BOSTON-1 on 24 Feb 2021
- 601 Kramer BOSTON-1 on 02 Mar 2021
- 701 Monforte BOSTON-2 on 03 Mar 2021 x2
- 107 Baz BOSTON-1 on 03 Mar 2021
Additional thanks to the following teams for their screening efforts:
- 701 Monforte
- 116 Huang
- 502 Gottlieb
- 706 Cifrian
- 301 Knoop
- 114 Arcasoy
- 110 Hachem
- 601 Kramer
How Do You Like the Newsletters?
Feedback (positive or constructive) is welcome at boston.sitesupport@zambongroup.com.
Help Needed, Questions or Suggestions?
The development program for inhaled L-CsA for the treatment of bronchiolitis obliterans is very important to Zambon.
LabCorp is responsible for the operational conduct of the studies and your LabCorp contact should be your first point of contact for questions.
However, please do not hesitate to get in touch with us directly if you’d like. Emails addressed to boston.sitesupport@zambongroup.com will reach the Zambon study team and will be answered promptly.
Newsletters are for BOSTON clinical site personnel only and not intended to be shared with patients, trial participants or any external audiences.
