
BT-L-CsA-301-SLT
BT-L-CsA-302-DLT
BT-L-CsA-303-FU
NEWSLETTER NO. 15
Introducing the New (US) BOSTON-1 & -2 Study Website!
Recently, a study website for the BOSTON-1 and -2 studies was launched in the US. The website provides brief information about BOS, clinical trials in general and the BOSTON studies. The website can be found at www.studyforbos.com
For the time being, the website is for healthcare professionals in the US only. However, the website is currently being translated and the European country sites will follow soon. Eventually, we plan to make this a “public” website, that can be shared with patients and families.
Investigator Meetings
Thank you to everyone who attended the Investigator meetings that took place on March 25, 29 & 30th. Covance will share the slide deck for your reference and, for those who could not attend, recordings of the meeting will be available (reach out to your CRA).
Frequently Asked Questions – Diagnosis of CLAD-BOS
The diagnosis of BOS is based on two main pillars: (i) sustained decline of FEV1 to ≤80% and (ii) ruling out other causes for deterioration of pulmonary function.
We have received many questions regarding the date of CLAD-BOS onset and date of CLAD-BOS diagnosis, the latter being important for patient eligibility. The date of CLAD-BOS onset is defined in the ISHLT guidelines published by Verleden and colleagues, as “the date at which the first value of FEV1 ≤80% of baseline is recorded after a diagnosis of BOS has been confirmed”.
The date of diagnosis of CLAD-BOS is the date when a clinical diagnosis of BOS is confirmed by the treating physician (this date can be different from the date of CLAD-BOS onset). According to the study protocol and per ISHLT recommendations, a sufficient diagnostic proof of diagnosis of CLAD-BOS is using – amongst others – spirometry, body plethysmography, and/or CT scans. The date of diagnosis should be available in the patient’s medical records. This date should be used for the eligibility check and used for the entry in the eCRF “Date of first BOS diagnosis”.
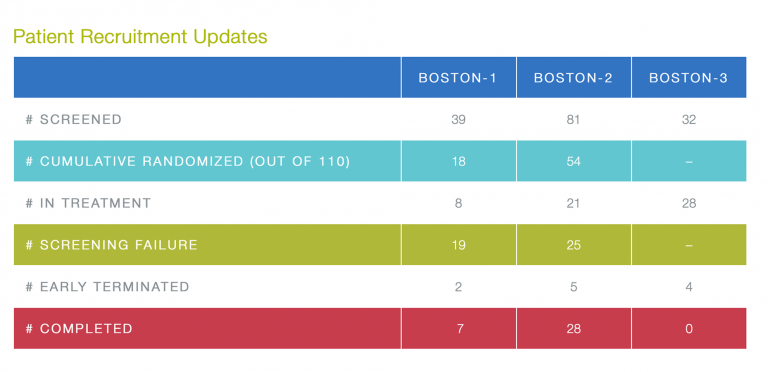
Kudos for new randomizations go to the following sites:
- 601 Dr. Kramer, SC Hilda Levi and team
- 107 Dr. Baz, SC Leah Woods and team
- 701 Dr. Monforte, SC Sonia Lopez and team
- 110 Dr. Hachem, SC Hannah Perkins and team
- 706 Dr. Cifrian, SC Pilar Alonso and team
- 116 Dr. Huang, SC Ibrahim Abukenda and team
- 109 Dr. Pilewski, SC Carol Oriss and team
- 301 Dr. Knoop, SC Anne-Marie Salumu and team
- 112 Dr. Hays, SC Reed Norris and team
How Do You Like the Newsletters?
Feedback (positive or constructive) is welcome at boston.sitesupport@zambongroup.com.
Help Needed, Questions or Suggestions?
The development program for inhaled L-CsA for the treatment of bronchiolitis obliterans is very important to Zambon.
LabCorp is responsible for the operational conduct of the studies and your LabCorp contact should be your first point of contact for questions.
However, please do not hesitate to get in touch with us directly if you’d like. Emails addressed to boston.sitesupport@zambongroup.com will reach the Zambon study team and will be answered promptly.
Newsletters are for BOSTON clinical site personnel only and not intended to be shared with patients, trial participants or any external audiences.
