
BT-L-CsA-301-SLT
BT-L-CsA-302-DLT
BT-L-CsA-303-FU
NEWSLETTER NO. 17
Zambon recently attended the virtual ISHLT meeting on April 24-28, 2021.
“Our activities at ISHLT demonstrate our commitment to serving the undertreated area of severe respiratory diseases. These are diseases that continue to have significant impacts on patient outcomes and healthcare systems. Zambon presentation and posters at ISHLT 2021 speak to the urgency to find a solution for one such disease – BOS. We have seen research showing that the development of BOS within 5-years post-transplantation remains unfortunately high and also the significant economic burden associated with this condition. Such findings reiterate the importance of the work we are doing in the ongoing BOSTON studies – and our other programs in severe respiratory diseases – to deliver meaningful treatments to patients whose options are currently limited,” said Paola Castellani, CMO and R&D Head at Zambon.
You can view the full press release discussing the economic impact of BOS presented at ISHLT here.
At the meeting, Dr. Ajay Sheshadri of the Department of Pulmonary Medicine, University of Texas MD Anderson Cancer Center, Houston, TX, presented on Bronchiolitis Obliterans Syndrome Following Lung Transplantation: Economic Burden by Chronic Lung Allograft Dysfunction (CLAD) Stage.
Two posters authored by Zambon staff were accepted for presentation at the meeting:
(i) Emilie Hofstetter and Gerhard Boerner presented their research on the “Development in Lung Transplantation, Organ Shortage, Bronchiolitis Obliterans and Overall Survival in the USA, 2011-2018” (abstract 754); and
(ii) Stefanie Prante Fernandes presented on behalf of the BOSTON-1 & -2 study team on how the studies were adapted and managed in response of the COVID-19 pandemic: Ensuring Patient Safety and Data Integrity in Clinical Trials for the Treatment of Bronchiolitis Obliterans Syndrome (BOS) during the COVID-19 Pandemic (abstract 780).
Thank you to everyone who was able to attend the oral and poster presentations. For those of you who were unable to attend, you can now view these on our website here.
In addition…
We will have virtual booths at the upcoming ATS meeting, May 14-19, 2021 and the AARC summer forum in July. More information to come.
Patient Recruitment Updates
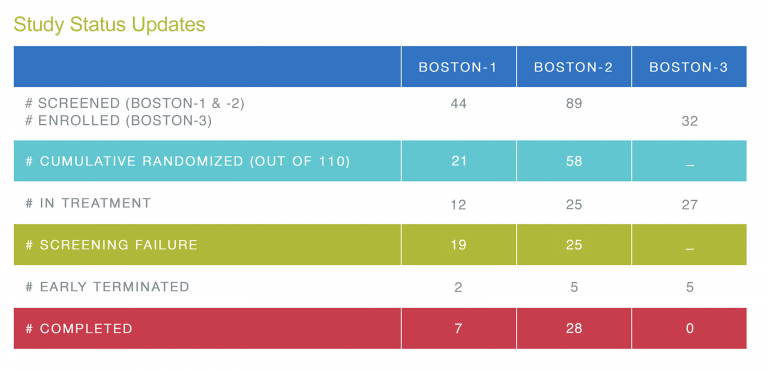
BOSTON-2 enrollment 50% complete!
We are excited to announce that on 19 April 2021 subject #55 was enrolled in BOSTON 2 bringing enrollment to 50%. A special thank you to all investigators, study coordinators and their teams for helping to achieve this important milestone. Let’s keep this momentum going for 2021!
Kudos
Kudos for new randomizations go to the following sites:
- 701 Dr. Monforte, SC Sonia Lopez and team
- 116 Dr. Huang, SC Ibrahim Abukenda and team
- 112 Dr. Hays, SC Reed Norris and team
- 114 Dr Arcasoy, SC Leonor Suarez and site team
Study Steering Committee
We are happy to announce that we have established a Study Steering Committee of 3 experienced investigators who will help us as advisors on the conduct of the BOSTON-1, -2- and -3 studies. This will include but is not limited to: (i) Impact of new events (e.g. COVID-19, new scientific data, new/competitive studies), (ii) Recruitment (study, country and site level), and (iii) Facilitate communication where needed and exchange of experience among sites.
The committee consists of:
- Selim Arcasoy, New York, USA (site 114)
- Jens Gottlieb, Hannover, Germany (site 502)
- Victor Monforte Torres, Barcelona, Spain (site 701)
How Do You Like the Newsletters?
Feedback (positive or constructive) is welcome at boston.sitesupport@zambongroup.com.
Help Needed, Questions or Suggestions?
The development program for inhaled L-CsA for the treatment of bronchiolitis obliterans is very important to Zambon.
LabCorp is responsible for the operational conduct of the studies and your LabCorp contact should be your first point of contact for questions.
However, please do not hesitate to get in touch with us directly if you’d like. Emails addressed to boston.sitesupport@zambongroup.com will reach the Zambon study team and will be answered promptly.
Newsletters are for BOSTON clinical site personnel only and not intended to be shared with patients, trial participants or any external audiences.
