
BT-L-CsA-301-SLT
BT-L-CsA-302-DLT
BT-L-CsA-303-FU
NEWSLETTER NO. 20
eFlow Nebulizer Randomization Checklist
The randomization visit (V1) is a busy day, both for study patients and site teams, with many different assessments and activities throughout the visit. Based on our experience with more than 100 patients (with more than 50 patients randomized to the treatment arm), we created a list of key points or reminders for the randomization visit. It can be used as a resource during assigning and setting up the devices or instructing study participants during their first L-CsA treatment.
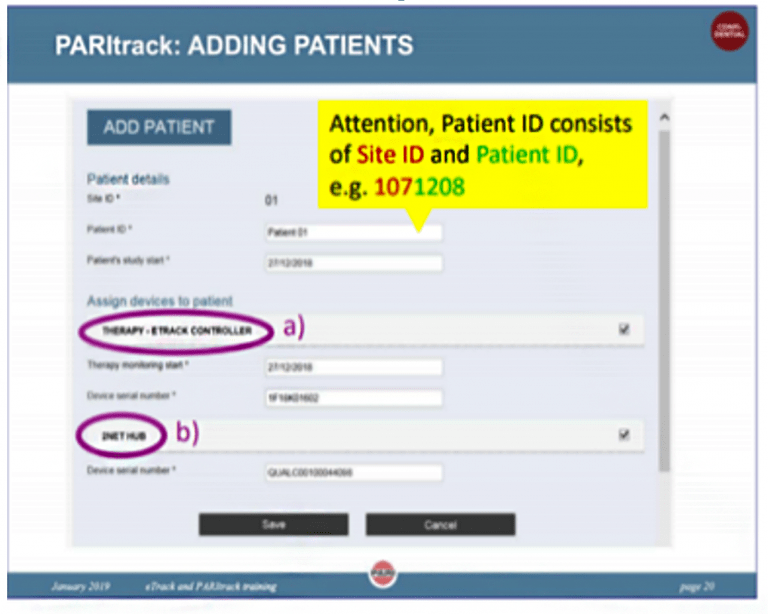
- Assign the eTrack Controller and 2Net hub in PARItrack web portal prior to the first L-CsA inhalation; When adding a new patient to PARItrack web portal use: site ID + patient ID, e.g., 1071208
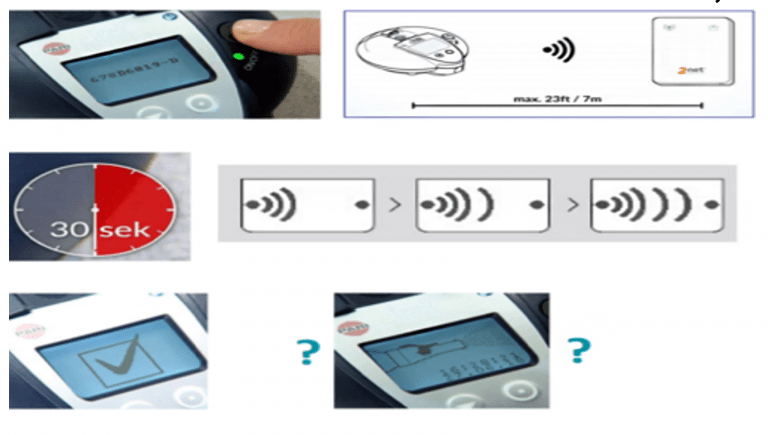
- Activate the 2Net hub by plugging it into an electrical outlet.
- Switch ON the eTrack controller, it will automatically pair with the 2Net hub.
- Be careful with the aerosol head, do not touch the metal aerosol membrane.
- Do not shake the L-CsA vial while dissolving, to prevent foaming.
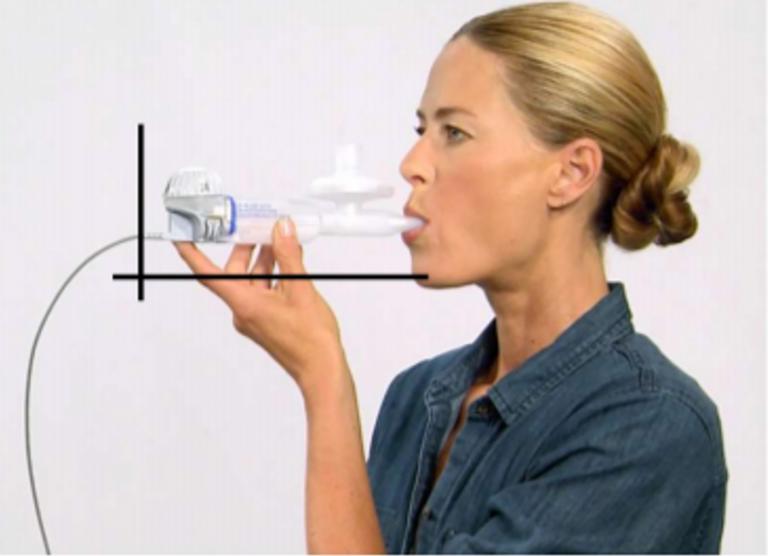
- Instruct your participants to hold handset at level and to Inhale slow and deep through mouthpiece.
- Remind your participants to check medication reservoir after treatment, use easycare or a new aerosol head for replacement, if more than a drop remains as residual volume.
- Remind your participants to rinse, clean, disinfect all handset parts after each treatment; especially cleaning with clear liquid dish soap prevents clogging of the aerosol head by drying L-CsA lipids.
- For additional device support or training, please reach out to your designated CRA or to us directly (boston.sitesupport@zambongroup.com). We are happy to walk you through all the steps until you are fully comfortable, especially in case of a first patient or site personnel new to the study.
Frequently asked questions
Question: A potential study patient was initially diagnosed with BOS grade 0-p (within 12 months after transplantation), which later progressed to BOS grade 1. Which date should be considered for assessing inclusion criterion 3 “Diagnosis of CLAD-BOS must be made at least 12 months after lung transplantation […]”. Is such a patient eligible?
Answer: According to current consensus (Verleden et al. 2019), CLAD is defined as “a substantial and persistent decline (≥20%) in measured FEV1 value from the reference (baseline) value”. The guidance requires a persistent drop of lung function of (≥20%). However, the date of onset of CLAD is defined “as the date at which the first value of FEV1 ≤80% of baseline is recorded.”
However, the guidance also does acknowledge that a drop in FEV1 of ≥10% can be considered outside the normal day-to-day variation and that further investigations should be started. After these assessments, certain patients may present with clinically defined BOS even though their drop in FEV1 did not (yet) reach ≥20%. The study protocol does allow inclusion of these patients with an FEV1 range of greater 80% up to 85% of baseline, as long as these patients are diagnosed with clinically defined BOS and do meet all other inclusion and exclusion criteria of the study protocol. A drop of FEV1 of ≥10% alone does not qualify for a diagnosis of CLAD-BOS (this, amongst others, is a reason why the term BOS0-p has been removed from the guidance).
For a specific patient, the answer re. patient eligibility, often “depends” and will require a critical assessment of the patient by the investigator. The date of BOS diagnosis should then be documented in the source data in an unambiguous way.
BOSTON-2 upcoming interim analysis
You might have heard already from Labcorp (Covance’s new name) that there is an upcoming interim analysis of the study. Additionally, some of you might already have wondered why Labcorp is now pushing for data entry, monitoring of data, and answering data queries (and may be bothering you with timelines for this). In this short section, we would like to provide some background information and explain why this is important for the study.
When planning the sample size of a clinical trial, the researchers need to balance two types of intrinsic errors. The alpha error is detecting a treatment effect where there is none, and the beta error is about not detecting a treatment effect where there is one. For both of these errors, biostatisticians and regulators have defined conventions. When estimating the required sample size of a planned clinical trial, the researchers need to rely on assumptions for the measures supporting the primary analysis, which in BOSTON-2 include the standard deviation of FEV1 measurements. While previous and published data may give some guidance, the “real” data may be different.
The scientific and regulatory guidance does allow blinded(!) interim analyses to verify the initial planning assumptions with “real” study data, as long as this is specified upfront in the study protocol. If the “real” data should be different, this would allow the researchers to adjust the sample size of the study accordingly via a study protocol amendment.
The study protocol of the BOSTON-2 study does specify such interim analysis one-two months before the last patient is projected to be randomized. Thanks to your enrollment efforts, we will arrive there soon! However, the interim analysis will be reliable only if performed on “clean” data. This is the reason why Labcorp did already start pushing you for data entry, monitoring and data queries.
Kudos
Kudos for new randomizations go to the following sites:
- 701 Dr. Monforte, study coordinator Sonia Lopez and team (2 patients)
- 119 Dr. Dhillon, SC Emerald Mann and team
- 703 Dr. de la Torre, study coordinator Cristina Quiñones and team
- 502 Dr Gottlieb, Ilona Olzik and team (2 patients)
- 501 Dr. Jürgen Behr, Dr. Marion Frankenberger, Marlen Wenzel and team (2 patients)
- 112 Dr. Hayes, study coordinator Reed Norris, and team
How Do You Like the Newsletters?
Feedback (positive or constructive) is welcome at boston.sitesupport@zambongroup.com.
Help Needed, Questions or Suggestions?
The development program for inhaled L-CsA for the treatment of bronchiolitis obliterans is very important to Zambon.
LabCorp is responsible for the operational conduct of the studies and your LabCorp contact should be your first point of contact for questions.
However, please do not hesitate to get in touch with us directly if you’d like. Emails addressed to boston.sitesupport@zambongroup.com will reach the Zambon study team and will be answered promptly.
Newsletters are for BOSTON clinical site personnel only and not intended to be shared with patients, trial participants or any external audiences.
