
BT-L-CsA-301-SLT
BT-L-CsA-302-DLT
BT-L-CsA-303-FU
NEWSLETTER NO. 21
Dear BOSTON-1/-2 & -3 Study Teams,
In this edition of the study newsletter, we would like to provide you with information for device training, new data entry requirements for BOSTON-3, and update you on the current recruitment status and the upcoming interim analysis.
BOSTON-2 Interim Analysis
Many thanks for supporting the interim analysis of the BOSTON-2 study, especially for entering data and replying to data queries. The interim analysis does include all patients who have completed week 48 and, in addition, all patients, who have completed week 24. Currently, everything is on track. The results are scheduled to be available shortly and will tell us if the sample size of the study needs to be adjusted. We will keep you updated!
Device Training Refresher
The BOSTON clinical trials have been ongoing now for more than one and a half years. On this occasion and considering the potential of increasing need and high advantage, we would like to offer training refresher sessions to all site staff (participating in the BOSTON trials), especially to individuals providing ongoing support to patients, assigning devices in PARITrack web portal or performing device training to randomized patients.
Device Training gets even more important if your site has new staff members!
The training sessions can be provided onsite, depending on the current pandemic situation and traveling restrictions/regulations in your region, or alternatively on a remote basis (online).
For requesting training sessions, please reach out to your CRA.
BOSTON 3 New Data Entry requirements in Visit 1 - Informed Consent
With the implementation of study protocol version 2.0 of the BOSTON-3 study, the eCRF structure has been updated accordingly. In order to access the updated eCRF pages, entering the informed consent data as described below is required.
- If Subject is enrolled under BOS3 Protocol 1 and will switch to BOS3 Protocol 2, you need to enter re-consenting information in the Informed Consent page at Visit 1 (see below screenshots).
Please note that the updated CRFs pages will NOT be available until you enter the re-consenting data (yes or no).
Complete the new data points (shown in red boxes below):
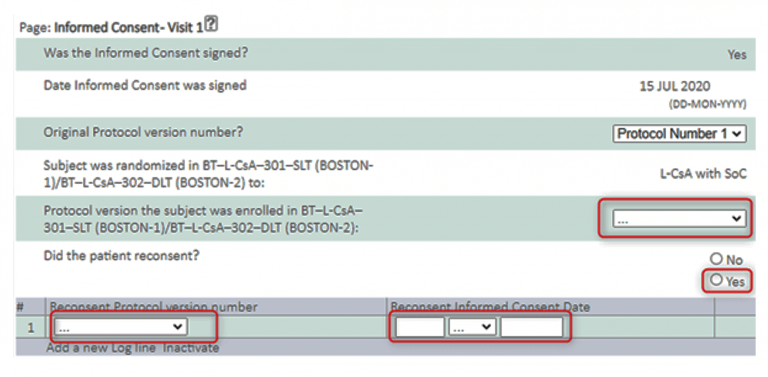
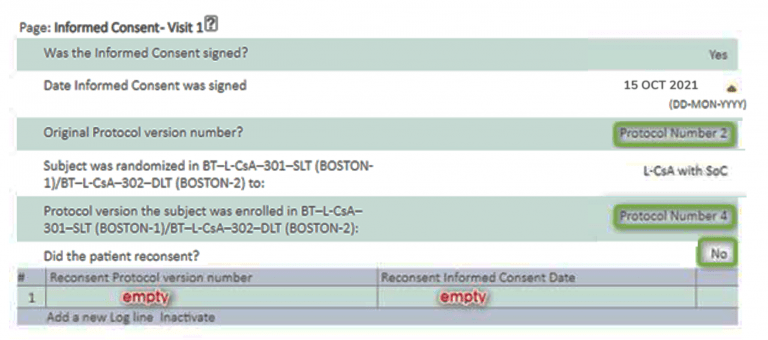
- If a Subject is not re-consenting because the patient was enrolled under protocol Version 2, the Informed Consent page at Visit 1 will still need to be completed, and completed as ‘No’.
Further Information:
The eCRF Completion guideline has also been updated and can be accessed directly via the EDC system.
Please find the complete guidance directly in you eCRF clicking on the help link.

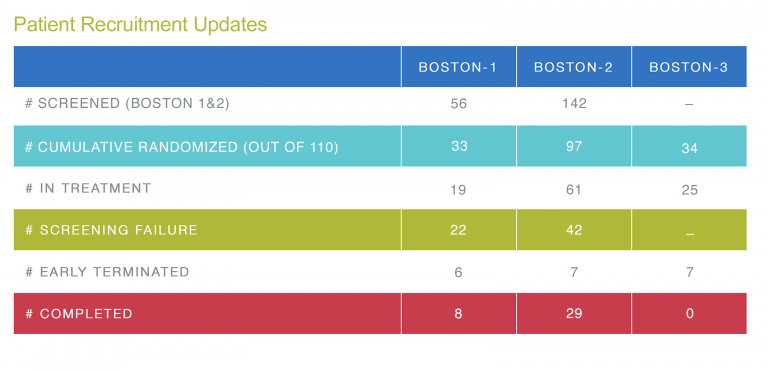
Patient Recruitment Updates
Recruitment into the BOSTON-2 study is progressing well. As you can see from the table below, the situation is different for the BOSTON-1 study. Here we are behind our planned schedule. Therefore, we would like to draw your attention also to the BOSTON-1 study and potential patients who may be eligible for the study.
Kudos
Kudos for new randomizations go to the following sites:
- 502 Dr. Gottlieb, Ilona Olzik and team for randomizing 4 patients to BOSTON 2.
- 501 Dr. Jürgen Behr, Dr. Marion Frankenberger, Marlen Wenzel and team for randomizing a patient to BOSTON 1 & 2.
- 112 Dr. Hays, SC Reed Norris and site team for randomizing 2 patients to BOSTON 2.
- 114 Dr. Arcasoy, SC Leonor Suarez and site team for randomizing a patient to BOSTON 1.
- 105 Dr. Hage, SC Jean Nash, and team for randomizing a patient to BOSTON 2.
- 101 Dr. Griffith, SC Maia Lee, and entire site team for randomizing a patient to BOSTON 2.
- 110 Dr. Hachem, SC Hannah Perkins, and entire site for randomizing a patient to BOSTON 2.
- 601 Dr. Mordechai Kramer, study coordinator Liora Yehoshua and site team for randomizing a patient to BOSTON 1.
- 118 Dr. Criner and study coordinators Heidi Shore Brown and Bob Burke for randomizing a patient to BOSTON 2.
- 116 Dr. Huang, study coordinators Joel Wilson, Lindsay Barter, and entire site team for randomizing a patient to BOSTON 2,
- 402 Dr. Martine Reynaud, study coordinators Sabrina Yaker, Yasmine Namouri and Genevieve Mouton for randomizing a patient to BOSTON 2.
How Do You Like the Newsletters?
Feedback (positive or constructive) is welcome at boston.sitesupport@zambongroup.com.
Help Needed, Questions or Suggestions?
The development program for inhaled L-CsA for the treatment of bronchiolitis obliterans is very important to Zambon.
LabCorp is responsible for the operational conduct of the studies and your LabCorp contact should be your first point of contact for questions.
However, please do not hesitate to get in touch with us directly if you’d like. Emails addressed to boston.sitesupport@zambongroup.com will reach the Zambon study team and will be answered promptly.
Newsletters are for BOSTON clinical site personnel only and not intended to be shared with patients, trial participants or any external audiences.
