
BT-L-CsA-301-SLT
BT-L-CsA-302-DLT
BT-L-CsA-303-FU
NEWSLETTER NO. 22
Dear BOSTON-1/-2 & -3 Study Teams,
In this edition of the study newsletter, we would like to highlight some attention that BOS (Bronchiolitis Obliterans Syndrome) and the BOSTON studies are receiving, provide you with some information on upcoming events, update you on the per protocol blind interim analysis, provide study reminders, and update you on the current recruitment status.
A Spotlight on BOS
BOS is gaining national attention in the US. The Lung Transplant Foundation is leading a Patient-Focused Drug Development (PFDD) meeting on June 22, 2022 for BOS. The meeting will be hosted in the Washington DC area, as well as in a virtual platform.
The PFDD meeting will give the FDA, medical product developers, and health care providers, an opportunity to hear directly from patients, their families, and caregivers about their BOS symptoms and unmet needs that matter most to them. Members from the Zambon team will be attending the meeting as silent observers. If you are planning to attend, we would love to hear from you.
Zambon will also be participating in the Rare Disease Week, ISHLT, ATS and the AARC conferences. More information to come.
Additionally, the BOSTON 1 & 2 studies were highlighted in the recent Pulmonary Fibrosis Foundation Clinical Trials Newsletter.
Interim Analysis Update
Increase BOSTON-2 Sample Size to 152 patients
During the recent per protocol blind Interim Analysis, the standard deviation re-estimated using the pre-defined linear mixed model (LMM) for the analysis of the primary endpoint was in line with the original protocol assumption. However, the same estimation based on the ANCOVA model led to the conclusion that a sample size increase from 110 to 152 subjects is needed to preserve the power of 80%. Based on the data collected at the time of the IA, the LMM is appropriate. Nevertheless, the Sponsor retains to increase the sample size to 152 subjects for the following two reasons:
-
Adequately power the study for ANCOVA, which may be necessary in case the LMM model is not viable at the end of the study, considering that COVID-19 pandemic is not resolved and might still have an impact on the number of dropouts and missing values
-
Improve the power of the sensitivity analyses envisaged by the protocol
We appreciate your continued effort and focus on enrollment for the BOSTON studies.
Study Reminders
BOS-1 & -2 Study Reminders
-
We are seeing a large number of system queries related to missing data on several forms. Data should be reported as soon as possible to avoid queries related to missing pages
-
There are a significant number of queries related to Lab results which are out of normal range requiring an assessment of clinical significance. Reporting the Clinically significant/ Not Clinically Significant assessment at the same time of lab values recording would reduce the queries
-
Please carefully check to ensure that Spirometry data (Date and Time of Assessment) filled in EDC will match the Date and Time of Assessment from COMPACT Spirometry transferred to Vitalograph
-
Please be diligent in keeping study supplies from different studies clearly separated and to pay attention in the completion of supplies accountability log.
BOS-3 Study Reminder
We would like to provide a reminder once again regarding EDC completion for the informed consent:
BOSTON 3 new Data Entry requirements in Visit 1 - Informed Consent
Please note that no new CRFs will be available until you enter the re-consenting data (yes or no).
- If Subject is under BOS3 Protocol 1 and will switch to BOS3 Protocol 2, you will need to enter re-consenting information in the Informed Consent page at Visit 1 (see below screenshots).
Complete the new data points (shown in red boxes below):
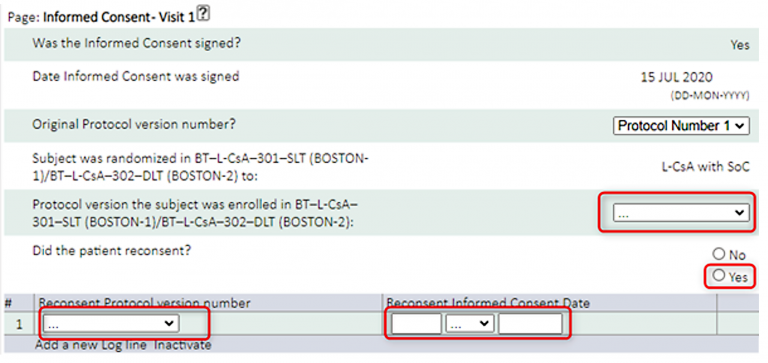
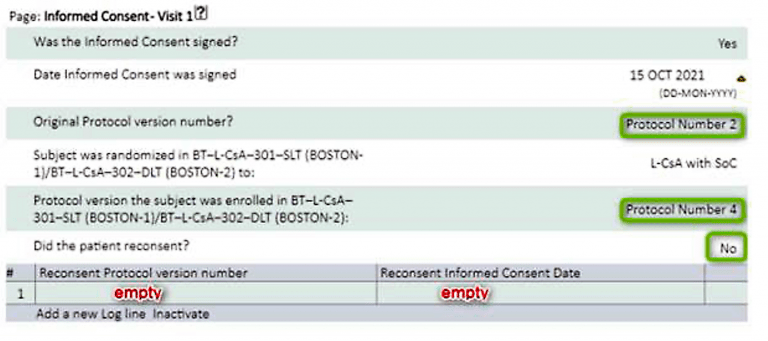
- If a Subject is not re-consenting at the next visit, the Informed Consent page at Visit 1 will still need to be completed, and completed as ‘No’.
Further Information:
Please find the complete guidance directly in you eCRF clicking on the help link
Current Recruitment
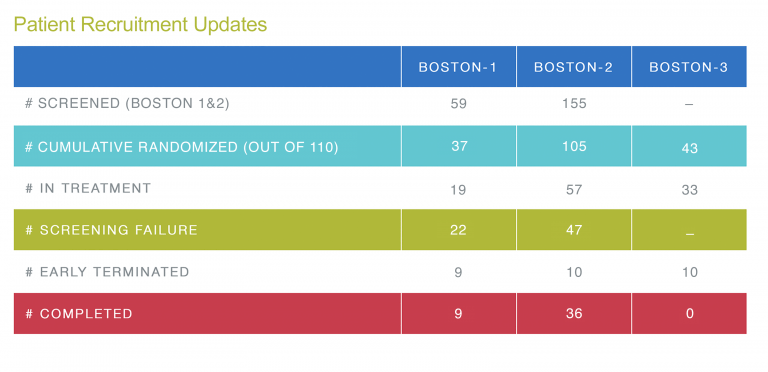
The current number of patients enrolled in BOS2 is 105, this is a great result, but considering the increase in the BOSTON-2 sample size to 152 patients another 47 patients need to be enrolled. We ask that you continue to focus on BOSTON-2 enrollment. It will be critical to reach the final target of 152 patients. As you can see from the table below, we are still behind our planned schedule for BOSTON-1. Therefore, we would like to draw your attention also to the BOSTON-1 patients who may be eligible for the study.
A look at enrollment in the US and EU
The graphs below show the current screening and randomization numbers in both the US and the EU countries.
Comparing the numbers of participating sites in each country, US sites have enrolled more patients in BOS-1 against EU sites which have screened and enrolled more suitable patients in BOSTON 2 Study.
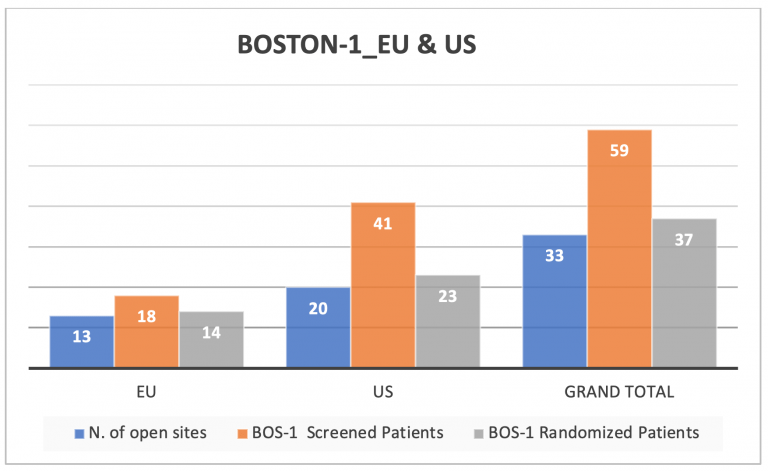
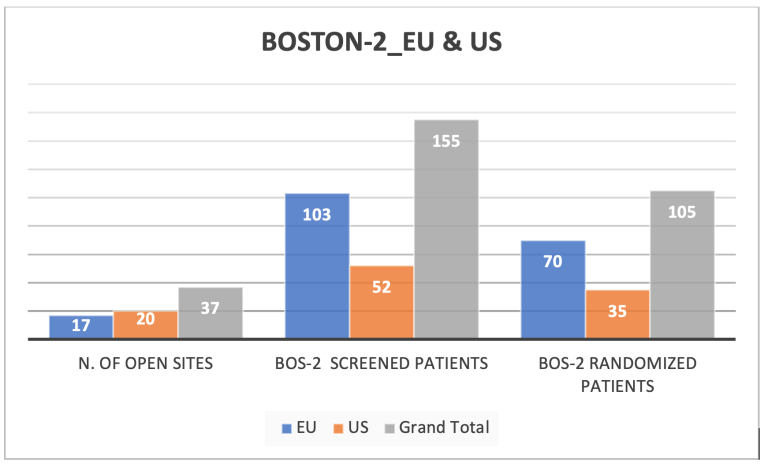
We are confident that all open sites, in US and EU, can actively contribute to study enrollment to reach the new study commitment of 152 patients in BOSTON-2 and increase the patients enrollment also in BOSTON-1.
Kudos
Kudos for new randomizations go to the following sites:
- Dr. Reynaud-Gaubert and SCs Yasmine Namouri, Genevieve Mouton and Sabrina Yaker at site 402 for their randomization to BOSTON-2.
- Dr. Jens Gottlieb, study coordinator Ilona Olzik and the team at site 502 for their randomization to BOSTON-2.
- Dr. Parmar and SCs Julie Zamikula and Sarah Dennis at site at site 801 for their randomization to BOSTON-1.
- Dr. Parmar and SCs Sarah Dennis, Julie Zamikula and Rebecca Mullen at site 801 for their randomization to BOSTON-2.
- Dr. Kramer and SC Liora Yehoshua at site 601 for their randomization to BOSTON-1.
- Dr. Jaksch and Frau Limbert at site 201 for their randomization to BOSTON-2.
How Do You Like the Newsletters?
Feedback (positive or constructive) is welcome at boston.sitesupport@zambongroup.com.
Help Needed, Questions or Suggestions?
The development program for inhaled L-CsA for the treatment of bronchiolitis obliterans is very important to Zambon.
LabCorp is responsible for the operational conduct of the studies and your LabCorp contact should be your first point of contact for questions.
However, please do not hesitate to get in touch with us directly if you’d like. Emails addressed to boston.sitesupport@zambongroup.com will reach the Zambon study team and will be answered promptly.
Newsletters are for BOSTON clinical site personnel only and not intended to be shared with patients, trial participants or any external audiences.
