
BT-L-CsA-301-SLT
BT-L-CsA-302-DLT
BT-L-CsA-303-FU
NEWSLETTER NO. 23
Dear BOSTON-1/-2 & -3 Study Teams,
In this edition of the study newsletter, we would like to provide study reminders, and to update you on the current recruitment status. We would also like to introduce new team members and to highlight some of our collaboration with patient advocacy groups. Lastly, we would like to share an update on our attendance at ISHLT and ATS, and to provide clarification on IMP device handling.
Current Recruitment
Boston-2 Study Recruitment Status
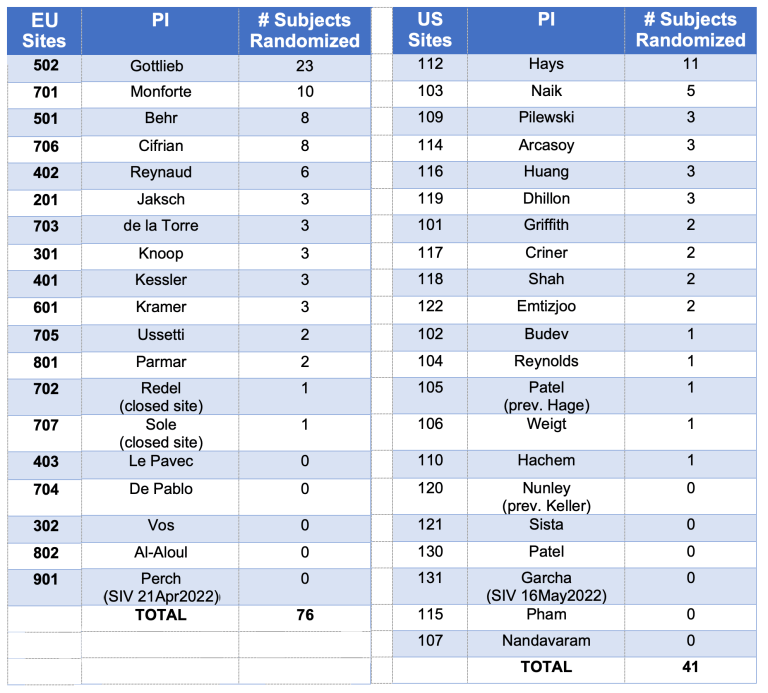
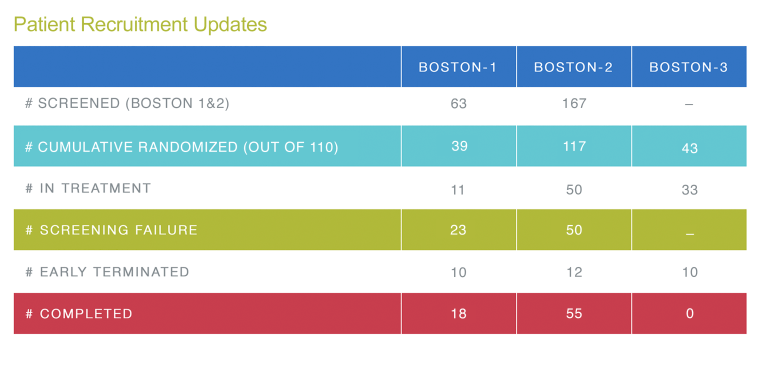
Up to now, 117 patients in the BOSTON-2 Study have been randomized. This is a great result – thanks to all of you for this valuable contribution.
Considering the increase of the sample size to 152 patients in the BOSTON-2 Study, another 35 patients will need to be enrolled by the end of September 2022.
Please continue to identify and screen subjects to help achieve this recruitment goal and please let us know if we can assist with these efforts in any way.
Following is the status of the enrollment for each of the participating sites:
BOSTON-1 Study Recruitment Status
The recruitment target for the BOSTON-1 Study remains 110 randomized patients. To date, 39 patients have been enrolled.
Please continue screening and enrollment efforts for this study as well.
BOSTON-1, BOSTON-2, BOSTON-3 Summary Recruitment Table
Kudos
Kudos for new randomizations go to the following sites:
- Dr. Jens Gottlieb, Study Coordinator Ilona Olzik and the team at site 502 for their randomization to BOSTON-2.
- Dr. de la Torre and Study Coordinator Cristina Quiñones at site 703 for their randomizations to BOSTON-1 and BOSTON-2.
- Dr. Huang, Study Coordinator Lindsay Barter, and the team at site 116 for their randomization to BOSTON-2.
- Dr. Dhillon, Study Coordinator Emerald Mann, and the team at site 119 for their randomization to BOSTON-2.
- Dr. Parmar and Study Coordinators Sarah Dennis, Julie Zamikula and Rebecca Mullen at site 801 for their randomization to BOSTON-2
- Dr. Kramer, Study Coordinator Hodaya Dekel and the team at site 601 for their randomization to BOSTON-1.
- Dr. Weigt, Study Coordinator Elman Punzalan, and the team at site 106 for their randomization to BOSTON-2.
- Dr. Criner, Study Coordinator Heidi Shore-Brown, and the team at site 117 for their randomizations to BOSTON-1 and BOSTON-2.
- Dr. John Reynolds, Study Coordinators Katelyn Arroyo and Lerin Eason, and the team at site 104 for their randomization to BOSTON-2.
- Dr. Budev, and Study Coordinators Joanne Baran and Hannah Johnson at site 102 for their randomization to BOSTON-2.
- Dr Monforte, Study Coordinator Sonia Lopez and the team at site 701 for their randomization to BOSTON-2.

Shahid J. Siddiqui, MD, Vice President, Medical Affairs
Shahid J. Siddiqui recently joined Zambon as Vice President, Medical Affairs. He has extensive global medical affairs, clinical development, and regulatory experience with over 25 years in global leadership roles at Astra Zeneca, Eli Lilly, Novartis, CHIRON, and most recently at Regeneron. At Regeneron, he led a team of MDs and MSLs with a focus on the upper and lower airways (Asthma, COPD, and CRSwNP). He also led global registries in Asthma and CRSwNP, and published many post hoc analyses from the company’s Asthma and CRSwNP pivitol studies.
During his tenure in clinical development as a clinical lead for Bevespi in COPD, Shahid was key to the successful regulatory submission of the NDA, as well as the pivotal trial execution, submission to the global health authorities, and its launches in US and globally. Prior to joining industry, Shahid worked as a clinical practitioner, having finished his residency in medicine at Jinnah Post Graduate Medical Center, Karachi, Pakistan. He also completed his Master's degree in Health Management at Ohio University. In his spare time, Shahid enjoys music and hiking during the summer months. His favorite TV channel is the Home Improvement station.

Michele DaSilva, MSEd, RRT, RRT-NPS, Device Trainer, USA
Michele recently joined the Clinical Service Team as Device Trainer for the US and brings exceptional experience to the team as a Respiratory Care Practitioner and Health Care Educator. In her last Clinical Liaison role Michele provided remote monitoring and education for patients with pulmonary diseases. She will be responsible for PARItrack Web Portal monitoring and documentation, internal and external eFlow device and PARItrack trainings. Michele works out of her home office in Stanhope, NJ, and in her spare time, she enjoys reading, gardening, cooking, and traveling. Michele is also a volunteer with Right2Breathe and the American Lung Association.
Patient-focused Drug Development Meeting
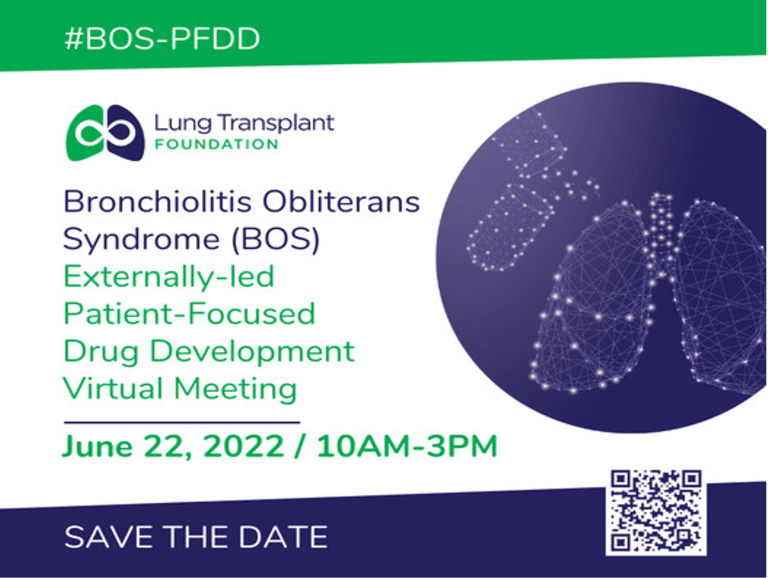
Collaboration with patient advocacy groups (PAGs) is of ongoing importance to our team and clinical programs. We have been working with the Lung Transplant Foundation (LTF) since the beginning of the BOSTON trials. The LTF has an upcoming initiative that we wanted to share. They are holding an externally-led, patient-focused drug development (PFDD) meeting on bronchiolitis obliterans syndrome (BOS). This virtual event will take place on June 22 at 10 AM – 3 PM EST. PFDD meetings provide the opportunity for patients and caregivers to share their stories with the United States Food and Drug Administration (FDA). These meetings allow the patient community to express unmet needs around their disease or condition. For rare diseases, like BOS, this is of the utmost importance as there are fewer voices to be heard.
The LTF would like all lung transplant recipients and others potentially impacted by BOS to listen in. The LTF also needs patients' stories. Individuals who would like to learn more or those who have stories to share, can follow the link below via the QR code or visit - https://lungtransplantfoundation.org/bos-pfdd/
Zambon Attendance at ISHLT and ATS Annual Meetings
During the ISHLT annual meeting, Zambon presented two poster presentations: Survival After Lung Transplantation in Europe - Chronic Lung Allograft Dysfunction (CLAD) as Major Cause of Death and Trend in Lung Transplantation in Europe 2015 - 2019, Type of Transplant and Organ Source. The company also exhibited in the Clinical Trials area at ATS 2022. It was a pleasure to meet some of you in Boston and San Francisco.
IMP Device Handling
Refresher on device assignment via PARItrack Web Portal
- CAVEAT: Wrong device for the appropriate study
- The Controller and 2net Hub devices differ between Boston-1/2 and Boston-3.
- Therefore, devices for Boston-1/2 cannot be used for Boston-3 and vice versa. Patients will receive new devices when rolling over to Boston-3.
- NOTE: If you can’t find the corresponding serial numbers in the drop-down menu when assigning the devices in the PARItrack, the devices do not belong to the appropriate study.
- If this occurs, check to ensure you have the correct device.
- If you still experience issues, please contact us for help (see below).
PARItrack Assignment – 3 step procedure for new randomized or rolled over patient. The order is important.
- Step 1 - Add a new patient to PARItrack and assign a Controller and 2net Hub to the corresponding patient.
- Step 2 - Plug the 2net Hub into a wall-socket and wait until the 2net Hub is ready for use.
- Step 3 - Switch on the eTrack Controller close to the 2net Hub (within a range of 7 meters/23 feet for pairing of the 2 devices).
The correct assignment of the devices is important to monitor data about the device use including technical and adherence data. Whenever you experience trouble with this, please contact us immediately for help.
- US: Michele DaSilva (michele.dasilva@zambongroup.com)
- EU: Salah Al Musawi (salah.al-musawi@zambongroup.com)
Study Reminders
BOS-1 & -2 & 3 Study Reminders
- EDC Completion. Reminder, please be sure to enter data in a timely manner. The study drug administration page must be completed promptly to ensure IMP stock consumption is reflected into RTSM and resupply is properly triggered.
How Do You Like the Newsletters?
Feedback (positive or constructive) is welcome at boston.sitesupport@zambongroup.com.
Help Needed, Questions or Suggestions?
The development program for inhaled L-CsA for the treatment of bronchiolitis obliterans is very important to Zambon.
LabCorp is responsible for the operational conduct of the studies and your LabCorp contact should be your first point of contact for questions.
However, please do not hesitate to get in touch with us directly if you’d like. Emails addressed to boston.sitesupport@zambongroup.com will reach the Zambon study team and will be answered promptly.
Newsletters are for BOSTON clinical site personnel only and not intended to be shared with patients, trial participants or any external audiences.
