
BT-L-CsA-301-SLT
BT-L-CsA-302-DLT
BT-L-CsA-303-FU
NEWSLETTER NO. 24
Dear BOSTON-1/-2 & -3 Study Teams,
In this edition of the study newsletter, we would like to provide FDA communications sent to you on August 8, 2022, update you on current recruitment, provide study reminders and inform you of recent and upcoming activities.
FDA Feedback
In the Written Response Only (WRO) received from FDA on 3-Aug-2022 the Agency recommended to continue BOSTON-1 and BOSTON-2 enrollment to strive to achieve the originally planned total number of 220 patients for both clinical trials combined, to ensure the adequacy of the safety database.
On August 8, 2022, a letter went out to the investigational sites to share recent FDA communications that resulted in a change to the recruitment strategy.
Given the current recruitment numbers for BOSTON-1 alongside the rarity and severity of bronchiolitis obliterans syndrome in SLTs patients, the possibility to reach a total number of 220 patients would be to continue the enrollment in both studies with BOSTON-2 going beyond the current target of 152 patients. This would result in a total sample of 220 patients for both clinical trials combined, as per the FDA recommendation.
Based on this consideration, we would like to confirm that the enrollment period for both studies will continue beyond September 2022 until the achievement of 220 patients on the two studies combined, expected to take place by March 31, 2023.
Labcorp team members will contact you to begin the amendments process.
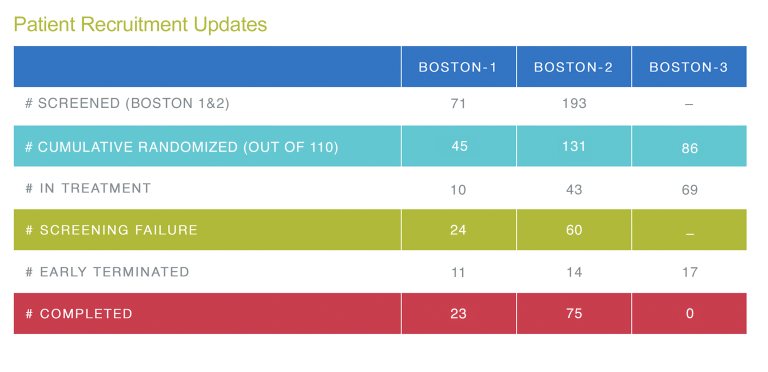
Current Recruitment
As noted above, the enrollment period for both studies will continue beyond September 2022 until the achievement of 220 patients on the two studies combined, expected to take place by March 2023. The current number of patients enrolled in both BOSTON-1 and BOSTON-2 is 174. We appreciate all of your continued hard work.
Kudos
Kudos for new randomizations go to the following sites:
- Dr. Arcasoy and the team at site 114 for their randomization to BOS 1
- Dr. Vos and the team at site 302 for their randomization to BOS 2
- Dr. Perch and the team at site 901 for their randomization to BOS 2
- Dr. Criner and the team at site 117 for their randomization to BOS 1
- Dr. Shah and the team at site 118 for their randomization to BOS 2
- Dr. Le Pavec and the team at site 403 for their randomization to BOS 2
- Dr. Ussetti and the team at site 705 for their randomization to BOS 2
- Dr. Arcasoy and the team at site 114 for their randomization to BOS 1
- Dr. Nunley and the team at site 120 for their randomization to BOS 2
- Dr. Santhanakrishnan and the team at site 802 for their randomization to BOS 2
- Dr. Cifrian and the team at site 706 for their randomization to BOS 1
- Dr. Huang and the team at site 116 for their randomization to BOS 1
- Dr. Jaksch and the team at site 201 for their randomization to BOS 2
- Dr. Budev and the team at site 102 for their randomization to BOS 2
- Dr. Sista and the team at site 121 for their randomization to BOS 2
- Dr. Monforte and the team at site 701 for their randomization to BOS 2
- Dr. Cifrian and the team at site 706 for their randomization to BOS 2
- Dr. Perch and the team at site 901 for their randomization to BOS 2
Study Reminders
We would like to take this opportunity to focus on the proper reporting of eligibility criteria in source documentation for clinical trials, according to the Q&A: Good clinical practice (GCP) | European Medicines Agency (europa.eu)
Please find here a short extract of the EMA Q&A question No. 4 “How can proper documentation of eligibility be ensured?”:
It is frequently seen during GCP inspections that the CRF is designed to only include an overall statement regarding a subject's eligibility in the trial. The text in the CRF could for instance say: 'Did the subject satisfy all study entry criteria?'. The statement is typically intended to be answered with 'yes' or 'no'.
The expectation of the GCP Inspectors' Working Group is that adherence to all individual inclusion and exclusion criteria are documented in the source data.
GCP inspections have revealed a substantial amount of cases where the overall eligibility statement in the CRF confirms subject eligibility but where source data shows that the subject did not fulfil all eligibility criteria. In addition, it has often not been documented that an investigator/sub-investigator has reviewed all criteria prior to inclusion. It therefore seems that a system with an overall statement in the CRF regarding a subject's eligibility in itself does not ensure the safety of the subjects, the quality of the data and sponsor oversight.
Please continue to be diligent in providing source documentation for each eligibility criteria. We have provided a checklist to assist in providing explicit review of eligibility criteria, but this checklist does not replace medical record source documentation to support eligibility for all criteria.
If you have any questions, please reach out to your CRA.
Upcoming Activities
Zambon will be attending the CHEST conference in Nashville, TN, October 16-19, 2022, Exhibit Booth #1223.
Zambon USA will be hosting a 45-minute non‐CME Learning Theatre Luncheon during CHEST on Tuesday, October 18, 2022, from 12:30 – 1:15 pm, Theater 4.
In addition to the learning theater, we will have a poster presentation by Emilie Hofstetter, Business Intelligence and Strategy: CHRONIC LUNG ALLOGRAFT DYSFUNCTION (CLAD): THE LEADING CAUSE FOR RETRANSPLANTATION ASSOCIATED WITH HIGH COST. We hope you will join on October 19, 2022 at 11:15-12:15pm.

World Lung Day September 25
Respiratory Care Week 2022 (Oct. 23–29) - A special thank you to all of our Respiratory Therapists.
The Lung Transplant Foundation is planning a BOS Awareness Day on October 25
How Do You Like the Newsletters?
Feedback (positive or constructive) is welcome at boston.sitesupport@zambongroup.com.
Help Needed, Questions or Suggestions?
The development program for inhaled L-CsA for the treatment of bronchiolitis obliterans is very important to Zambon.
LabCorp is responsible for the operational conduct of the studies and your LabCorp contact should be your first point of contact for questions.
However, please do not hesitate to get in touch with us directly if you’d like. Emails addressed to boston.sitesupport@zambongroup.com will reach the Zambon study team and will be answered promptly.
Newsletters are for BOSTON clinical site personnel only and not intended to be shared with patients, trial participants or any external audiences.
