
BT-L-CsA-301-SLT
BT-L-CsA-302-DLT
BT-L-CsA-303-FU
NEWSLETTER NO. 8
This newsletter represents the first in a short series of interviews of members of our BOSTON Clinical Program. In this interview, Jens Stegemann, PhD, CEO and Co-Founder of Breath Therapeutics, discusses the epidemiology and burden of Bronchiolitis Obliterans Syndrome (BOS).
Understanding the Disease and the Epidemiology

A CHAT WITH
Jens Stegemann
BOSTON Program
What is BOS and what causes it? Which are the risk factors?
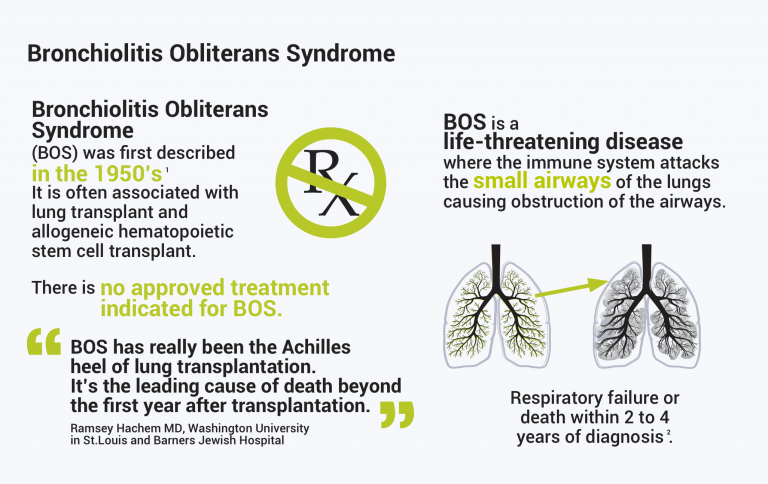
Bronchiolitis obliterans syndrome (BOS) is a rapidly progressive lung disease that usually leads to respiratory failure and death. It is caused by an inflammatory process triggered by the immune system that irreversibly destroys the airways of the lung. BOS commonly occurs in patients after lung and stem cell transplantation, and in other cases may develop as a result of autoimmune diseases or environmental exposure to harmful substances.
What are the clinical symptoms of BOS?
The clinical symptoms include a significant rapidly progressive and permanent reduction in lung function. BOS can be confirmed by CT scan, which visualizes the destroyed areas of the small airways. Reliable biomarkers for the diagnosis of BOS are currently not available.
Is there any geographic prevalence? Is BOS more prevalent in men or women?
There is no geographic or gender-specific prevalence in BOS. The prevalence, as a chronic post-transplant complication, is directly related to the number of lung and stem cell transplantation procedures. These procedures are mostly conducted in Western Europe and North America.
While Asian countries perform fewer lung transplants, they are conducting a growing number of stem cell procedures, so we expect to see more BOS there as well.
Do you have other interesting figures/data that you want to share on BOS epidemiology?
We believe the incidence of BOS is much higher than currently documented by registries, especially after stem cell transplantation, where screening for BOS is still very much underperformed. The other reason to believe BOS is under-reported is that there is no therapy available to treat BOS. If we are successful in developing an effective treatment, this could lead to an increase in awareness and fundamentally change the landscape of screening for and diagnosing BOS. Finally, BOS can also occur as a result of auto-immune diseases, infections and environmental exposure to harmful substances. These BOS figures are difficult to estimate so we believe we are only scratching the surface in terms of identifying the full burden of illness of BOS.
Why is Zambon’s work in BOS important?
The BOSTON development program is important to patients since there is currently no approved treatment indicated for BOS.
An estimated 50% of lung transplant patients develop BOS within five years after lung transplantation5 and this debilitating disease can lead to respiratory failure and death within two to four years after diagnosis.6 We endeavor to bring to patients the first safe and effective treatment indicated for BOS, and much hope to those who currently have no therapeutic options.
1. Gourtsoyiannis NC. Radiologic-Pathologic Correlations from Head to Toe. 2005: p.154
2. Chambers DC, et al. J Heart Lung Transplant. 2018;37(10):1169–1183.
3. Data on File, Breath Therapeutics, a Zambon company, 2019.
4. Benden C. Pediatric Lung Transplantation. J Thorac Dis. 2017.
5. Weigt SS, et al. Semin Respir Crit Care Med. 2013;34(3):336–351.
6. Chambers DC, et al. J Heart Lung Transplant. 2018;37(10):1169–1183.
How Do You Like the Newsletters?
Feedback (positive or constructive) is welcome at boston.sitesupport@zambongroup.com.
Help Needed, Questions or Suggestions?
The development program for inhaled L-CsA for the treatment of bronchiolitis obliterans is very important to Zambon.
LabCorp is responsible for the operational conduct of the studies and your LabCorp contact should be your first point of contact for questions.
However, please do not hesitate to get in touch with us directly if you’d like. Emails addressed to boston.sitesupport@zambongroup.com will reach the Zambon study team and will be answered promptly.
Newsletters are for BOSTON clinical site personnel only and not intended to be shared with patients, trial participants or any external audiences.
