
BT-L-CsA-301-SLT
BT-L-CsA-302-DLT
BT-L-CsA-303-FU
NEWSLETTER NO. 9
Status Update
With this edition of the newsletter, we would like to provide you with a brief update on the status of the BOSTON 1, 2 & 3 studies.
With the first approvals in place for the resumption of these studies, we started the re-opening of the sites in October and expect the majority of sites to be opened for patient recruitment before the Christmas holidays.
In many regions, the number of COVID-19 cases are going up again. In this “second wave”, we are well prepared and with the goal of not halting the studies again in the future. With the new amendments in place, all new patients will be equipped with home spirometers and visits from V2 can be performed remotely in the event of COVID-19 restrictions (see section 14.2.8 of study protocol).
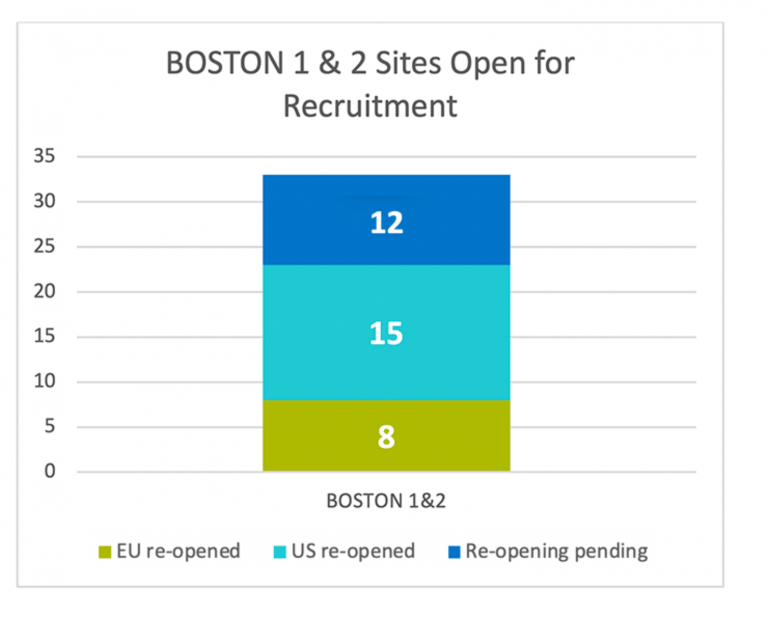
Site Re-Opening Status
The chart below shows the site re-opening status as of 04 December:
Pre-Screening log
A new version of the pre-screening log has been issued recently and shared with the sites. We encourage the use of this log as it may help you with the identification of patients. In addition, this will allow us to better understand the reasons for non-inclusion.
Considering the new protocol amendment just approved, it may be worth revisiting the “old” logs for previously excluded patients that now may qualify.
Patient Recruitment Status
Some patients are already being “pre-screened” or already in formal screening for BOSTON 1 & 2.
To the teams of Dr Cifrian Martinez at site 706 in Santander in Spain and of Dr Behr in Munich, “Kudos” for the first two “new” patients randomized after the re-start. Congratulations!
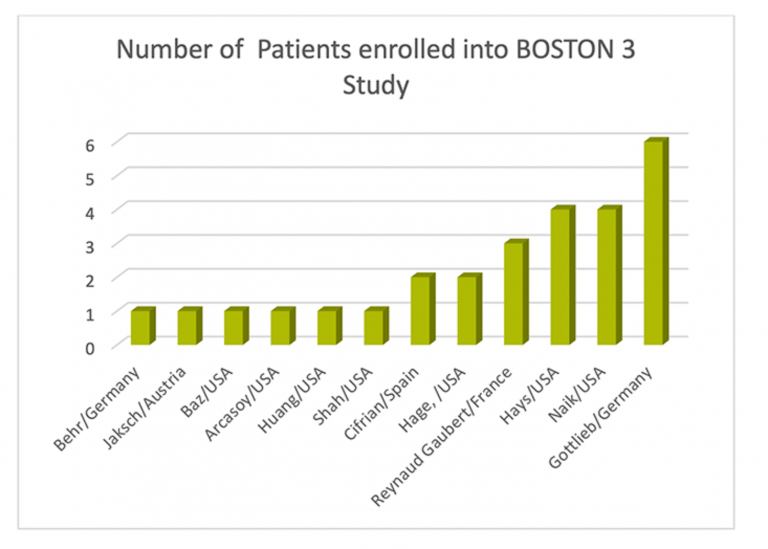
In addition, as noted in the chart below, the majority of patients already rolled over into BOSTON-3 during the course of the year. Many thanks for this!
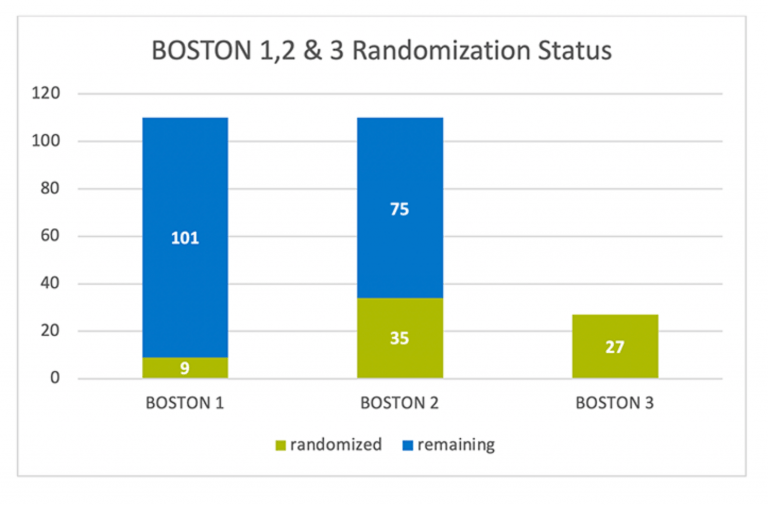
The chart below does show the current randomization status per study:
How Do You Like the Newsletters?
Feedback (positive or constructive) is welcome at boston.sitesupport@zambongroup.com.
Help Needed, Questions or Suggestions?
The development program for inhaled L-CsA for the treatment of bronchiolitis obliterans is very important to Zambon.
LabCorp is responsible for the operational conduct of the studies and your LabCorp contact should be your first point of contact for questions.
However, please do not hesitate to get in touch with us directly if you’d like. Emails addressed to boston.sitesupport@zambongroup.com will reach the Zambon study team and will be answered promptly.
Newsletters are for BOSTON clinical site personnel only and not intended to be shared with patients, trial participants or any external audiences.
